
How Does Global Warming Affect Biodiversity?
Plants, animals, bacteria, and fungiform are an ecosystem of our Earth. Despite being so rich, yet due to constant change
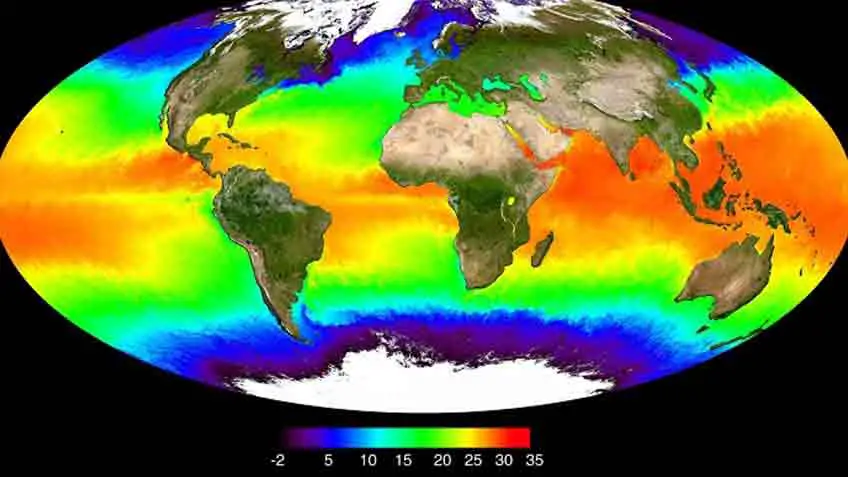
How Does Global Warming Affect Coral Reefs
The primary threat that coral reefs are confronting on a worldwide scale is climate change. Researchers concur that environmental change

When Do You Start Planting Flowers
It is essential to start planting flowers at the right time! But how would you know what is the right

How To Save Green Bean Seeds For Planting Next Year
Haven’t tried saving seeds? Saving green seeds can prove to be the best way to level up your gardening skills!

How Do Landfills Contribute To Global Warming
Have you ever thought about the waste you produce every day? Where does it go after the trash collector collects

How Does Recycling Help Global Warming
The waste that we produce every day brings a devastating impact on global warming and climate change in many ways.

When To Planting Potatoes
What comes to your mind first when someone asks you to name a vegetable used worldwide? However, it can be

How To Harvest Sunflower Seeds For Planting
Sunflower is known as a staple food supply for millions! It can be turned to make some stunning recipes like

How To Sprout Sweet Potatoes For Planting
Unlike most vegetables and fruits, sweet potatoes are sprouted from slips rather than seeds! This is something that makes their

How To Prepare A Flower Bed Before Planting
Do you have a garden that is overgrown with weeds? If yes, then you have landed over the right place

How To Prepare Soil For Planting Shrubs
Are you going to prepare the soil to plant shrubs for the first time in your garden? Then, you have

How to Save Spaghetti Squash Seeds for Planting
When it comes to squash you will find them in two major types, the spaghetti squash seed, and summer squash.

How To Make Seed Balls For Planting
Have you been exploring ways to enrich your skills in natural farming? If yes, then making seed bombs can help

How To Make A Planting Box From A Wood Pallet
Wooden pallets for plantations have become new-known for the past few years. Despite being elegant yet, wooden pallets are way

How Does Global Warming Affect Animals
No one can deny that the effects of climate change are not just limited to humans; the wildlife, including sea

What Planting Zone Is Austin Texas
Regardless of whether you are a farmer or gardener, you’ve most likely known about the United States Department of Agriculture’s

Pennsylvania Planting Zone
Pennsylvania is known for its diverse climatic landscape and geographic features. The huge planting area in this state comes with

What Planting Zone Is Chicago
In the gardening arena, you must have heard people talking about their zones, have you? The USDA hardiness zone is

How Does Global Warming Affect The Water Cycle
Climate change has a significant impact on the earth’s water resources. The warming atmosphere has changed water patterns from oceans

Difference Between Global Warming & Climate Change
Have you been reading up on the impact of climate change and global warming? These two key terms are often

How To Prepare Garden Soil For Spring Planting
Healthy soil is a must-have element to acquire some stunning upshots from farming! Whether you are going to plant vegetables,

How To Dry Seeds For Planting
If you have heirloom plants or you are particularly fond of specific flowers in your garden, you can grow them

What Is The Spacing For Planting Corn
Have you ever observed a corn crop? Why do some farmers have wide rows and others have narrow rows? First

How To Prepare Seeds For Planting
Gardening has become a new norm to many living in cities too! But it seems pretty expensive to some of

What Fertilizer To Use When Planting Grass Seed
Growing brand new grass in your lawn demands significant attention and care to get you well-established grass. Fertilizers in this

Why Global Warming Is Not A Threat
For the past few decades, global warming has been a subject of discussion for scientists to decide if it exists

How Does Global Warming Affect The Desert?
We are more concerned about melting polar tundra when it comes to the effects of global warming. However, a rise

Why Do We Need To Stop Global Warming?
You can name it a hot debate of this century! From a frequent rise in annual temperature to excessive wildfires

How To Prepare For Global Warming
Global warming has an immense impact on climate change! From wildfire to heavy rainfall & temperature rise can be computed

Save Tomato Seeds
Are you the type of gardener who loves to save seeds for next season? Then you have got the right

What Zone Is Ohio?
To find out what zone Ohio is, you need to understand the USDA hardiness zone map. This map breaks up

How To Grow Rice?
Rice is one of the essential staples that is being used in almost every part of the world. It is

What Is Planting
Planting is a whole new process to germinate plants, trees, and crops, whether into a container or soil. But unfortunately,
